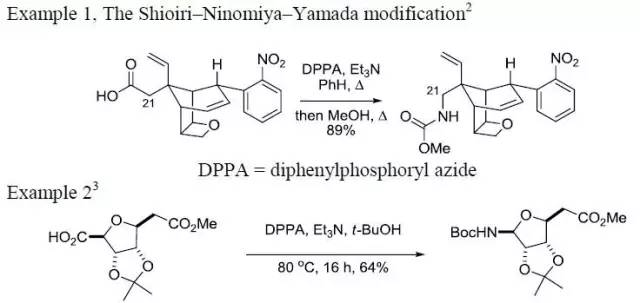
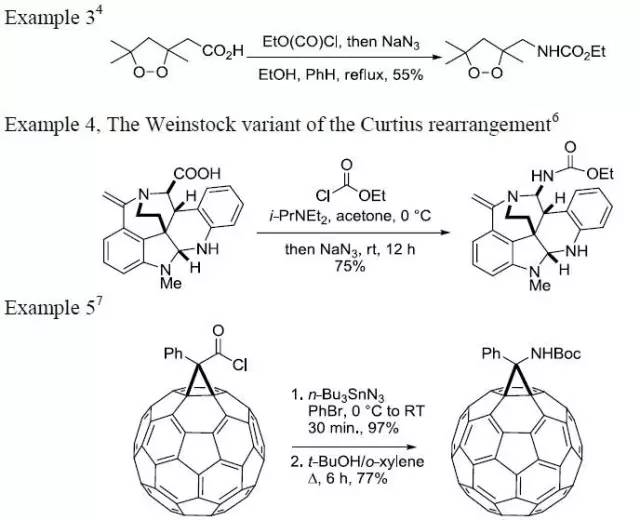
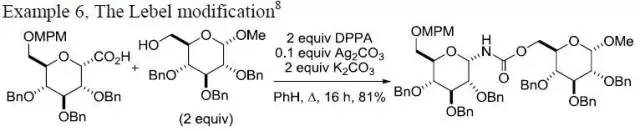
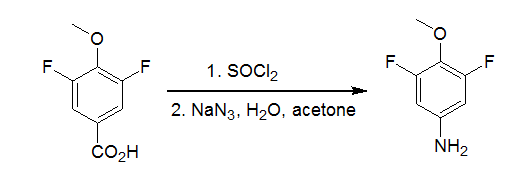| Curtius 重排 | 您所在的位置:网站首页 › 异氰酸根水解得到醇 › Curtius 重排 |
Curtius 重排
|
反应实例
相关文献 1. Curtius, T. Ber. 1890, 23, 3033-3041. Theodor Curtius (1857-1928) was born in Duisburg, Germany. He studied music before switching to chemistry under Bunsen, Kolbe, and von Baeyer before succeeding Victor Meyer as a Professor of Chemistry at Heidelberg. He discovered diazoacetic ester, hydrazine, pyrazoline derivatives, and many nitrogen-heterocycles. Curtius also sang in concerts and composed music.【Theodor Curtius (1857-1928)生于德国杜伊斯堡。他在做化学研究之前是学音乐的,先后在跟随Bunsen, Kolbe, von Baeye和Victor Meyer做化学研究,后来出任海德堡大学教授。他发现了重氮乙酸酯,肼,吡唑啉衍生物和很多氮杂环化合物。虽然是个化学家,他仍然进行音乐创作和音乐会歌唱。】 2. Ng, F. W.; Lin, H.; Danishefsky, S. J. J. Am. Chem. Soc. 2002, 124, 9812–9824. 3. van Well, R. M.; Overkleeft, H. S.; van Boom, J. H.; Coop, A.; Wang, J. B.; Wang, H.; van der Marel, G. A.; Overhand, M. Eur. J. Org. Chem. 2003, 1704–1710. 4. Dussault, P. H.; Xu, C. Tetrahedron Lett. 2004, 45, 7455–7457. 5. Holt, J.; Andreassen, T.; Bakke, J. M.; Fiksdahl, A. J. Heterocycl. Chem. 2005, 42,259–264. 6. Crawley, S. L.; Funk, R. L. Org. Lett. 2006, 8, 3995–3998. 7. Tada, T.; Ishida, Y.; Saigo, K. Synlett 2007, 235–238. 8. Sawada, D.; Sasayama, S.; Takahashi, H.; Ikegami, S. Eur. J. Org. Chem. 2007, 1064–1068. 9. Rojas, C. M. Curtius Rearrangements. In Name Reactions for Homologations-Part II; Li, J. J., Ed.; Wiley: Hoboken, NJ, 2009, pp 136163. (Review). 10. Koza, G.; Keskin, S.; Özer, M. S.; Cengiz, B.; Şahin, E.; Balci, M. Tetrahedron 2013, 69, 395–409. 编译自:Name Reactions (A Collection of Detailed Reaction Mechanisms), Jie Jack Li, Curtius rearrangement,page 188-189. 酰基叠氮重排合成胺示例
2,6-difluoro-4-methoxyphenyl carboxylic acid (2.00 g, 10.6mmol) was dissolved in thionyl chloride (16 mL). One dropof DMF was added and the mixture was heated to reflux for 2 h. The crude mixture was evaporated to dryness and the residue was dissolved in 5mL acetone. A solution of sodium azide (970mg, 14.9 mmol) in water (2mL ) was added dropwise at room temperature. After 30 min, water (10 mL) was added and the solution was extracted with toluene (50 mL). The organic layers were dried over sodium sulfate and heated to reflux for 30 min. Then 10mL of a 45% sodium hydroxide solution was added and the mixture was heated for a further 30 min. The organic layer was separated, dried over sodium sulfate and evaporated. The residue was purified by column chromatography (dichloromethane) to yield 660 mg (39%) of the title compound. Reference:Tetrahedron Lett., 2004, 45, 95 - 98. 使用DPPA合成胺示例
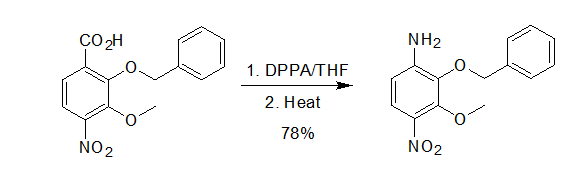
2-benzyloxy-3-methoxy-4-nitroanilin acid (27.9 g, 91.8 mmol) was dissolved in THF (400 mL) and treated with Et3N (30 mL). Diphenylphosphoryl azide (26.5 g, 96.4 mmol) was added dropwise and the reaction mixture was stirred for 3 h at 25 oC. H2O (150 mL) was added and the reaction mixture was refluxedfor 2 h. The solvent was removed in vacuo and the residue was treated with saturated aqueous K2CO3 (100 mL), diluted with H2O (500 mL), and extracted with EtOAc (2 × 500 mL). The combined organic extracts were washed with saturated aqueous NaCl (500 mL), dried (Na2SO4), and concentrated in vacuo. The crude residue was purified by flash chromatography (SiO2, 25% EtOAc−hexanes) to afford the title compound (19.5 g, 78%) as a yellow solid. Reference:J. Am. Chem. Soc., 2004, 126,8396 - 8398. 使用DPPA和苄醇合成Cbz保护的胺示例
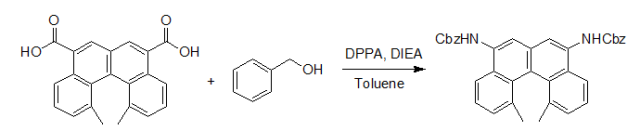
Under an argon atmosphere, amixture of acid(200 mg, 0.59 mmol), diisopropyl ethylamine(0.36 mL, 2.0 mmol), diphenylphosphoryl azide (0.32 mL, 1.5mmol) in toluene (25 mL) was heated at reflux for 3 h. Afterbeing cooled to room temperature, benzyl alcohol (0.2 mL, 2mmol) was added, and the mixture was stirred for another 1h. After removing the solvent in vacuo, silica gel columnchromatography gave the title compound (230 mg, 0.50 mmol,85%). Reference:J. Org. Chem.,2001, 6, 557 - 563. 使用DPPA和叔丁醇合成Boc保护的胺示例 由于叔丁醇的活性不高,一般都使用叔丁醇作溶剂,在研究过程中我们发现若在反应液中加入3-5当量的Boc2O可抑制副反应,提高反应产率。
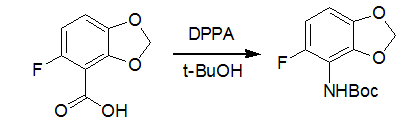
Dry tert-butyl alcohol (123 mL), triethylamine (16.7 g, 0.65mol), and DPPA (45.5 g, 0.165 mol) were added to a solutionof 5-fluoro-1,3-benzodioxole-4-carboxylic acid (29 g, 0.157mol) in dioxane (430 mL) under nitrogen. The mixture washeated at 100 °C for 4.5 h. Upon cooling, the cloudy mixturewas filtered. The filtrate was evaporated under vacuum,diluted in ethyl acetate, washed with a 5% aqueous citric acid,a 5% aqueous sodium bicarbonate, water, and brine, dried overmagnesium sulfate, and concentrated under vacuum to providedesired compound (37.6 g, 93%). Reference:J. Med. Chem. 2004, 47, 871-887返回搜狐,查看更多 |
【本文地址】