| 酰胺降解制备腈也是个人名反应?von Braun酰胺降解反应 | 您所在的位置:网站首页 › schmidt重排机理 › 酰胺降解制备腈也是个人名反应?von Braun酰胺降解反应 |
酰胺降解制备腈也是个人名反应?von Braun酰胺降解反应
|
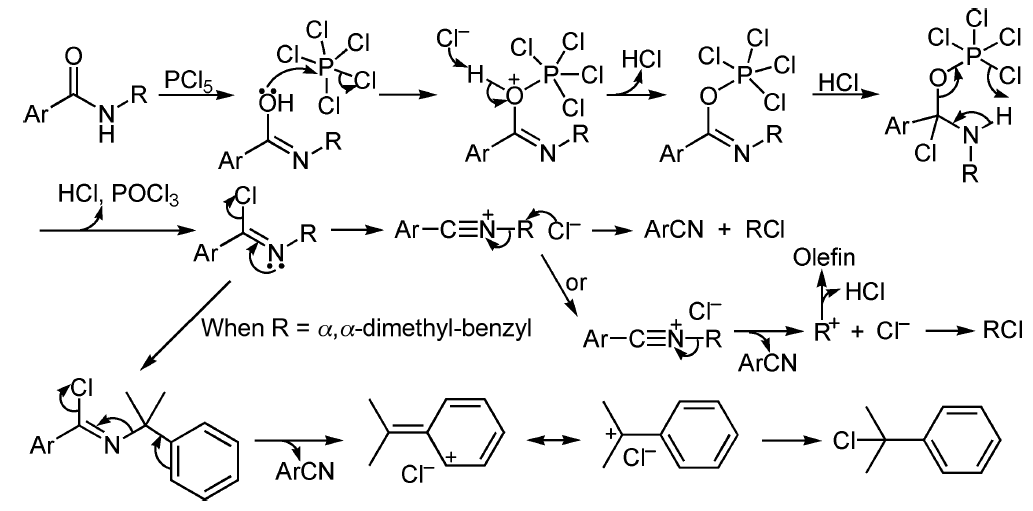
原标题:酰胺降解制备腈也是个人名反应?von Braun酰胺降解反应 N-烷基苯甲酰胺(N,N-二烷基苯甲酰胺或不含α-H的酰胺)和五卤化磷加热得到腈和卤代烷烃的反应,被称为von Braun酰胺降解反应。此反应最早由von Pechmann在1895年首先报道,后来von Braun在上世纪初对此反应进行系统的研究,因此此反应被命名为von Braun酰胺降解反应(和另外一个 von Braun反应 相区别)。此反应的底物通常是芳基甲酸的酰胺和不含α-H的酰胺,因为不含α-H的酰胺可能通过Bedoukian卤代生成α-卤代酰胺。电子效应和空间位阻都会影响此反应的产率。非苄基的N-烷基酰胺通过形成腈正离子中间体(nitrilium ion,Ritter中间体),接着卤离子亲核进攻中间体的α N-碳,得到腈,此反应相当于 Ritter反应 的逆反应。伯酰胺脱水制备腈相对容易【 酰胺脱水制备腈 】,条件温和, Burgess reagent [Et3N+SO2N-COOMe],三氟醋酸酐(TFAA)-三乙胺,(COCl)2-NEt3-DMSO,三聚氯氰等条件可以在低温和几乎中性的条件下反应,因此此反应合成价值较低,可以用于分析副反应,特别是对于 Ritter反应 , Schmidt重排 和 Beckmann重排反应 等涉及 腈正离子中间体的反应。但【 由叔丁酰胺制备腈 】效果还是很好的。 反应机理 酰胺通过五氯化磷脱水得到亚胺酰氯中间体,消除一个Cl离子生成腈正离子,Cl-亲核进攻 αN-碳得到腈和卤代烃。
反应实例 【 J. Am. Chem. Soc. , 1962, 84, 769】 【 J. Am. Chem. Soc. , 1949, 71, 2808】 A solution of 1.240 mmol ofN-tert-butyl-4-(6,7-dihydro-5H-[2]pyridin-7-yl)benzamide and 1.0 ml of thionylchloride in 30 ml of chloroform is stirred under reflux for 6 hours. The reaction mixture is cooled to roomtemperature and evaporated. The residueis taken up in dichloromethane and mixed with saturated aqueous sodiumbicarbonate solution. The organic phaseis separated and the aqueous phase is extracted with dichloromethane (2x). The combined organic phases are dried withsodium sulphate and concentrated. Theresidue is dissolved in diethyl ether and the title compound is converted intothe hydrochloride salt by adding ethereal HCI solution (2N). The solid is stirred in diethyl ether/acetone(1: 1), filtered and dried. The titlecompound is obtained as a dark grey solid. R f (free base) = 0.36 (EtOAc) 展开全文
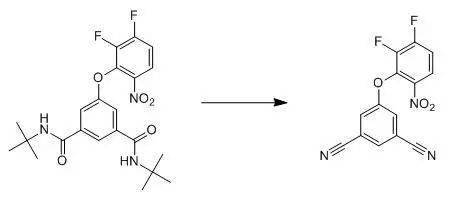
A 5 L round bottom flask was charged with N,N'-di- tert -butyl-5-(2,3-difluoro-6-nitro- phenoxy)-isophthalamide (21; 564 g) and 1.3 L ofphosphorus oxychloride. The mixture washeated to between 90 deg C. ~ 100.deg. C. for 2 h, after which approximately1/2 of the POCl3 was removed by distillation. Toluene was added (1 L) and additional liquidwas distilled. After cooling the mixtureovernight, a crude was obtained by filtration. Additional material was obtained by recovery from the mother liquid. The combined solids were stirred in MeOH (0.7L) for between 1 and 3 h, filtered and dried in a vacuum oven between 50~80 degC.at 25 Torr with a nitrogen bleed to afford 339 g of 22 (90percent theory). At ice-water bath, oxalyl chloride (0.345 ml) wasadded dropwise to a solution of 1.0 g of ethyl1-{4-[2-(t-butylaminocarbonyl)phenyl]phenyl}methyl-4-(1-hydroxy-1-methylethyl)-2-propylimidazole-5-carboxylate in 10 ml of methylene chloride. The mixture was stirred at the sametemperature for 2 hours. The reactionmixture was diluted with an aqueous solution of sodium hydrogencarbonate andethyl acetate, and the ethyl acetate layer was separated, dried over anhydrousmagnesium sulfate and concentrated by evaporation under reduced pressure. The residue was purified by silica gel columnchromatography, using 1:1 EtOAc/hex (v/v) as the eluent, to give 0.69 g of thetitle compound as crystals. 参考资料 相关反应 Ritter反应 Beckmann重排反应 Schmidt重排 酰胺脱水制备腈 返回搜狐,查看更多 责任编辑: |
【本文地址】