| 2.3: Isotopic Abundance and Atomic Weight | 您所在的位置:网站首页 › 毕拼音是什么 › 2.3: Isotopic Abundance and Atomic Weight |
2.3: Isotopic Abundance and Atomic Weight
|
Measuring Isotopic Abundances
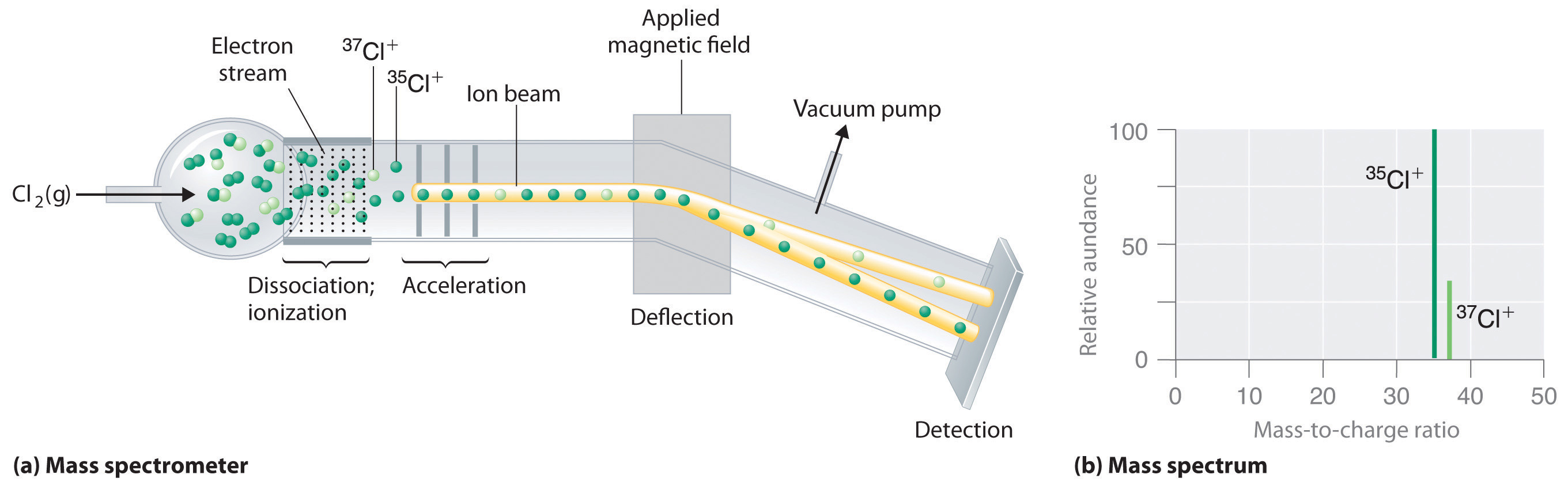
Although we cannot directly measure the mass of atoms, we can use Mass Spectrometer, an instrument that allows us to measure the mass to charge ratio. In figure 2.3.2 you can see chlorine gas entering an mass spectrometer. The chlorine has multiple isotopes and is hit with a stream of ionizing electrons which break the bond of Cl2 and strips electrons off the chlorine causing ions to form. These are then accelerated down the chamber until they reach a magnetic field that deflects the particles. The angle of deflection depends on both the mass of the particle and the magnetic field strength, with the lighter particles being deflected more (the lighter 35Cl+ ions are deflected more than the heavier 37Cl+ ions.) At the end of the chamber is an exit hole with a detector, and as the magnetic field intensity is increased the deflection angle changes, which separates the particles. Note, the mass spectrum in figure 2.3.2 (b) gives the relative abundance of each isotope, with the peak normalized to the isotope with the highest abundance. So if this ratio was 3:1 that means there are 3 particles of 35Cl for every particle of 37Cl, and the percent abundance would be 75% 35Cl and 25% 37Cl.
Figure 2.3.2 Determining Relative Atomic Masses Using a Mass Spectrometer Below is a video from YouTube describing the mass spectrometer
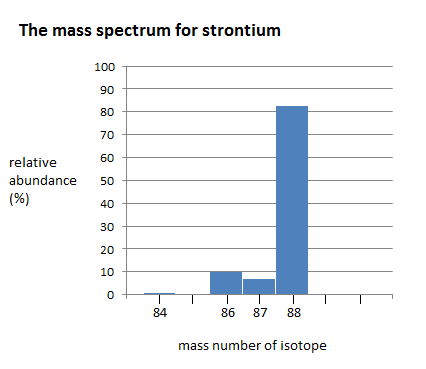
Here is a bar chart showing the relative abundance of 4 isotopes of strontium Example:
The mass spectrum of strontium has four different peaks, varying in intensity. The four peaks indicate that there are four isotopes of strontium. The four isotopes of strontium have isotopic mass numbers of 84, 86, 87, and 88, and relative abundances of 0.56%, 9.86%, 7.00%, and 82.58%, respectively. The intensity of the peak corresponds to the abundance. \(^{84}Sr\) has the smallest peak, which corresponds to its relative abundance of 0.56%, whereas \(^{88}Sr\) has the largest peak, which corresponds to its relative abundance of 82.58%. This indicates that \(^{88}Sr\) is the isotope that occurs in highest amounts.
|
【本文地址】